Why Bispecific Antibodies Plus CELMoDs Could Be a Powerful New Strategy in B-Cell Lymphoma
Adam Blum
Dec 22, 2025
Clinical trials don’t exist in isolation. They are built on layers of biology, prior evidence, and increasingly complex combinations of therapies. Trial NCT05169515 is a perfect example: a scientifically sophisticated study combining bispecific antibodies with next-generation immunomodulatory agents (CELMoDs) in relapsed or refractory B-cell non-Hodgkin lymphoma (NHL).
This is exactly the kind of trial that is extremely difficult to surface using simple keyword searches — and exactly the kind of trial that CancerBot’s precision trial-matching approach was designed to uncover.
What Is Trial NCT05169515?
NCT05169515 is an early-phase clinical study evaluating combinations of:
CD20×CD3 bispecific antibodies
Mosunetuzumab
Glofitamab
Cereblon E3 ligase modulators (CELMoDs)
CC-220 (iberdomide)
CC-99282 (golcadomide)
in adults with relapsed or refractory B-cell non-Hodgkin lymphoma (which includes my condition of FL) who have exhausted standard treatment options.
The study focuses on safety, tolerability, pharmacokinetics, and early signals of efficacy, with the broader goal of testing whether immune-engaging therapies can be made more potent and more durable through rational combination design.
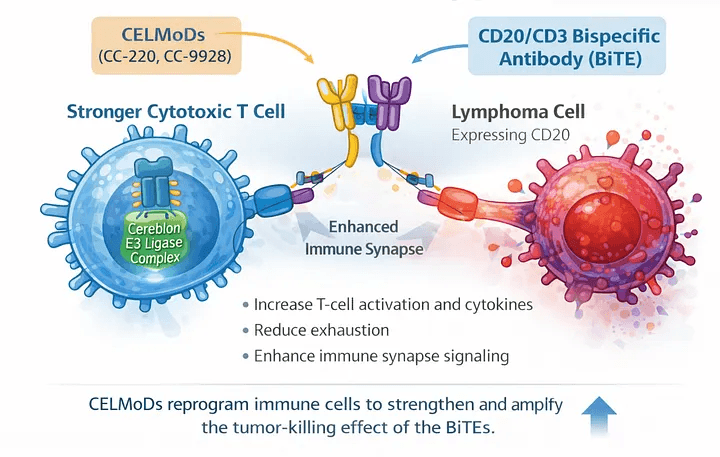
Why Bispecific Antibodies Are So Powerful — and Why They Still Need Help
Bispecific antibodies have transformed the treatment landscape for B-cell malignancies.
Agents like mosunetuzumab and glofitamab are engineered to bind:
CD20 on malignant B cells
CD3 on T cells
This physically forces a cytotoxic immune synapse to form — bringing T cells directly to the cancer cell and triggering tumor killing.
However, bispecifics rely on a critical assumption:
That the patient’s T cells are functional enough to respond.
In heavily pretreated lymphoma patients, that assumption is often shaky. T cells may be:
Exhausted
Numerically depleted
Suppressed by the tumor microenvironment
This is where CELMoDs come in.
CELMoDs: Turning Up the Immune System’s Volume
CELMoDs (cereblon E3 ligase modulators) are next-generation agents related to — but more potent than — classic IMiDs like lenalidomide.
Drugs such as CC-220 and CC-99282 work by binding cereblon and promoting degradation of transcription factors like IKZF1 (Ikaros) and IKZF3 (Aiolos).
The downstream effects include:
Enhanced T-cell activation
Increased cytokine production
Improved immune effector function
Direct anti-tumor effects in B-cell malignancies
In short, CELMoDs don’t just “support” the immune system — they re-engineer the immune environment to be more responsive.
Why the Combination Is More Than the Sum of Its Parts
The rationale behind NCT05169515 is not additive — it’s synergistic.
1. Bispecifics create the targeting signal
They bring T cells directly to lymphoma cells.
2. CELMoDs strengthen the killers
They improve the quality, durability, and intensity of the T-cell response once engagement occurs.
3. Dual pressure on the tumor
While bispecifics drive immune-mediated killing, CELMoDs may also exert direct biological pressure on malignant B cells — reducing the chance that resistant clones survive.
This combination is especially attractive in relapsed/refractory disease, where immune dysfunction and clonal evolution are major barriers to durable response.
This Strategy Already Has Proof-of-Concept
While bispecific + CELMoD combinations are still emerging, the broader strategy has already demonstrated success.
Bispecific + IMiD combinations
IMiDs are the predecessors of CELMoDs, and they’ve already validated the concept.
epcoritamab + R² (rituximab + lenalidomide)
→ Phase 3 data in follicular lymphoma showed superiority over R² alone.Mosunetuzumab + lenalidomide
→ Advanced to late-stage trials based on strong efficacy signals in relapsed follicular lymphoma.Glofitamab + lenalidomide
→ Active studies across multiple B-cell lymphoma subtypes with encouraging response rates.
These successes set the stage for CELMoDs — which are designed to be more potent immune modulators than lenalidomide — to potentially push outcomes even further.
NCT05169515 is testing that next evolutionary step.
Why Finding This Trial Is Hard Without Precision Matching
This is not a trial you find by typing:
“lymphoma immunotherapy trial”
To identify NCT05169515, you need to understand:
That it’s a combination study
That it involves bispecific antibodies (not CAR-T, not monoclonals)
That CELMoDs are distinct from IMiDs
That it targets relapsed/refractory B-cell NHL, not myeloma or leukemia
That multiple drug permutations exist within a single protocol
Traditional search tools treat trials as documents.
CancerBot treats them as structured biological objects.
By extracting:
Therapeutic modality
Molecular targets
Immune mechanism
Disease context
Line-of-therapy intent
CancerBot can surface trials like NCT05169515 that would otherwise be buried among thousands of loosely related studies.
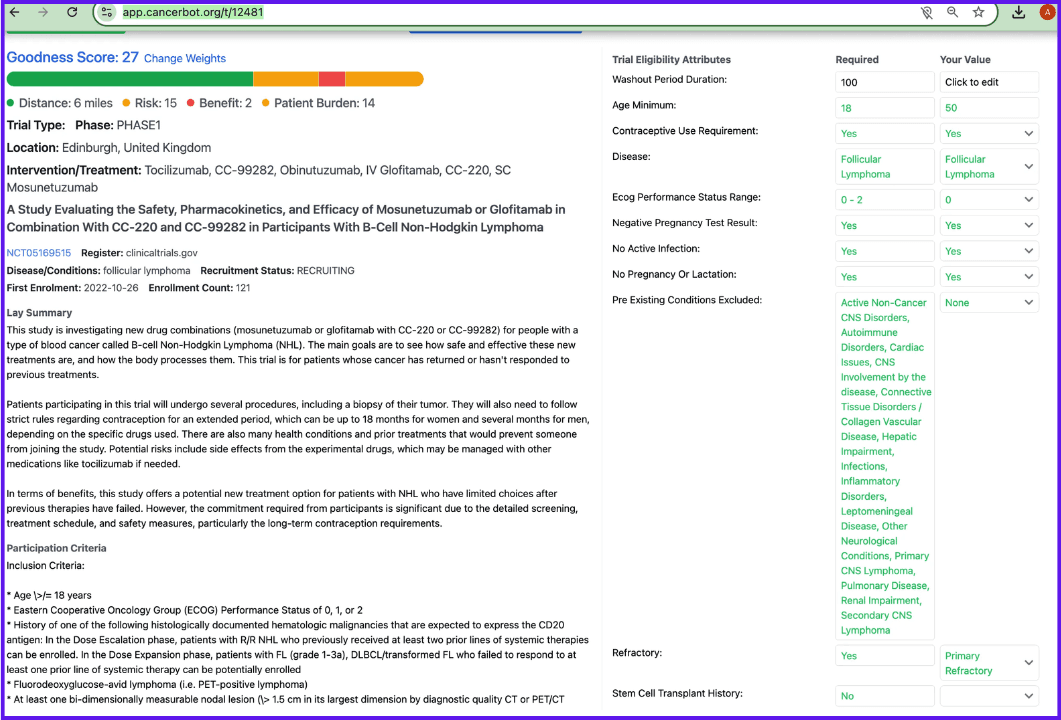
Here is what this trial looks like on CancerBot (specifically compared to my own diagnostics and previous FL treatment):