SYMPHONY-1: Can Adding Tazemetostat to R² Improve Outcomes in Relapsed Follicular Lymphoma?
Adam Blum
Dec 28, 2025
Clinical trials are the engine driving innovation in cancer care — especially in diseases like follicular lymphoma (FL) where cures are rare and durable remission is elusive. SYMPHONY-1 (NCT04224493) is one such trial that aims to push the boundaries of therapy by adding a targeted agent, tazemetostat, to an already effective immunochemotherapy backbone.
What Is SYMPHONY-1?
SYMPHONY-1 (also known as EZH-302) is a Phase 1b/3, double-blind, randomized, active-controlled trial evaluating whether adding an oral targeted agent, tazemetostat, to the immunomodulatory regimen lenalidomide + rituximab (R²) improves outcomes compared with R² alone in adults with relapsed or refractory FL who have had at least one prior systemic therapy.
The study is designed with:
A Phase 1b safety run-in to define the recommended Phase 3 dose (already completed), and
A Phase 3 randomized portion comparing tazemetostat + R² vs placebo + R² in ~500 patients worldwide. Health Research Authority
Why This Matters
FL is typically responsive to treatment but not curable with standard therapy. Many patients relapse or become refractory after frontline regimens. R² is a commonly used second-line therapy, combining:
Rituximab: antibody targeting CD20
Lenalidomide: immunomodulatory therapy
Adding a targeted agent like tazemetostat — a first-in-class oral EZH2 inhibitor — could enhance anti-tumor activity. Tazemetostat is already FDA-approved for certain relapsed FL patients.
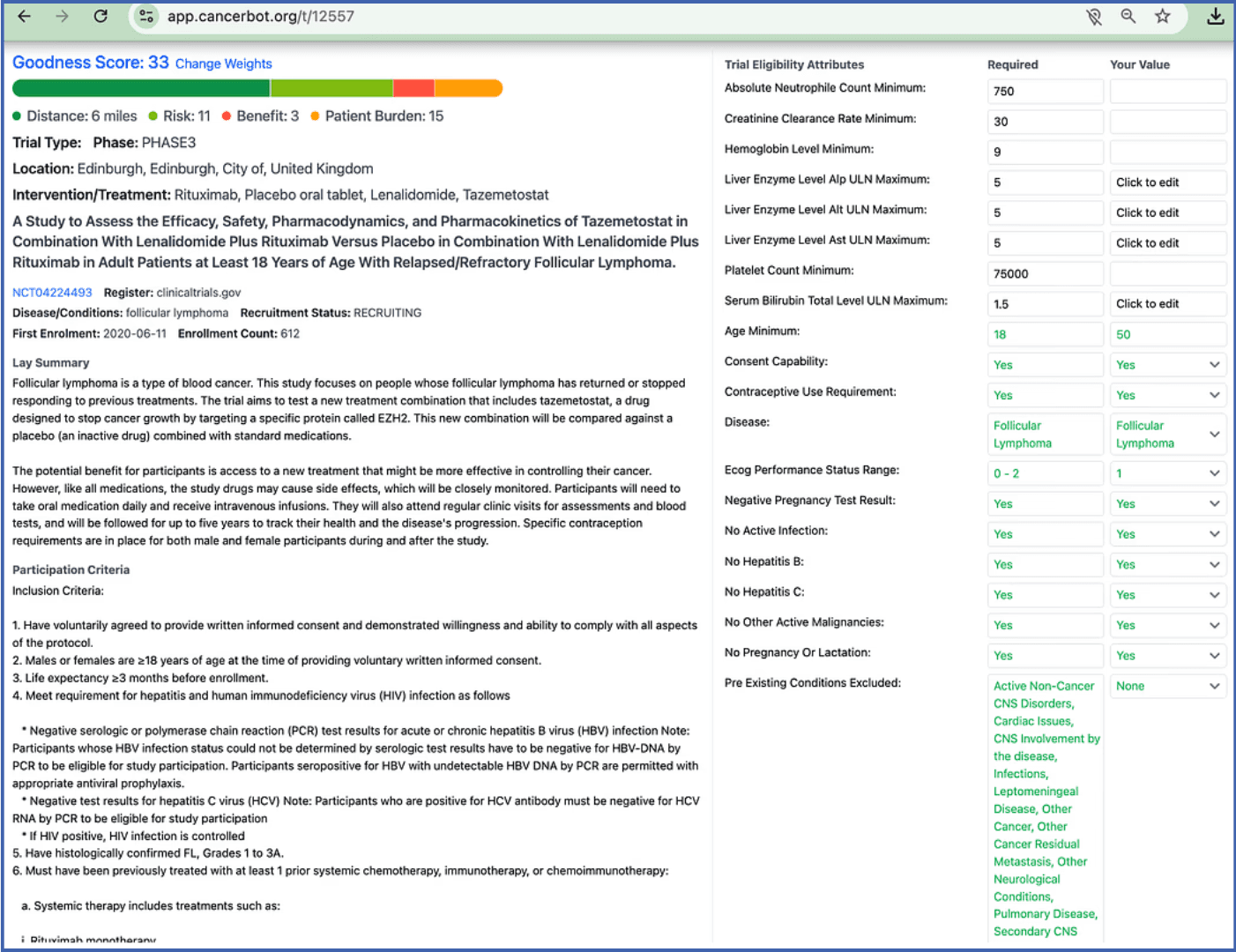
SYMPHONY-1 On CancerBot
What Has the Trial Shown So Far?
Although the Phase 3 portion is ongoing, the Phase 1b safety run-in provided encouraging early signals:
Recommended Phase 3 Dose: Tazemetostat at 800 mg twice daily alongside standard R².
High Response Rates: In the safety run-in cohort (44 evaluable patients), the overall response rate (ORR) was ~91%, with complete responses ~55% and partial responses ~41%.
Responses were seen regardless of EZH2 mutation status and even in high-risk subsets such as rituximab-refractory disease or early progression (POD24).
Median follow-up (~22.5 months) showed that median progression-free survival (PFS) and duration of response (DOR) had not yet been reached, suggesting durability.
These early signals are hypothesis-generating and helped confirm that the combination is tolerable and biologically active, supporting the ongoing randomized evaluation.
Why Target EZH2 in FL?
EZH2 is an epigenetic regulator often mutated or dysregulated in FL. It plays a role in suppressing tumor-suppressor genes and helping cancer cells thrive. Tazemetostat inhibits EZH2 — reversing some of those effects and potentially making the lymphoma more susceptible to immune-mediated and cytotoxic effects.
In SYMPHONY-1, investigators hope this targeted mechanism will enhance the effectiveness of R² beyond what either alone can achieve, especially in patients whose disease has progressed after prior treatment.
Who Might Consider This Trial?
SYMPHONY-1 is enrolling adults with relapsed or refractory FL who:
Have had at least one prior systemic therapy, and
Are eligible for lenalidomide + rituximab therapy as standard care. Lymphoma Action
Because it includes a placebo vs tazemetostat arm, eligibility requires willingness to be randomized and commit to the trial schedule, including follow-up visits for:
Infusions (rituximab)
Oral medications (lenalidomide + tazemetostat/placebo)
Regular monitoring (labs, imaging)
What Could This Mean for Patients?
If the Phase 3 portion shows a significant benefit in PFS (and potentially overall survival or quality of life), SYMPHONY-1 may establish a new standard of care in relapsed/refractory FL — adding a targeted agent to an already effective regimen for a deeper, more durable response.
Even if benefits are modest, understanding how tazemetostat interacts with immune-based therapy at different biological levels improves our ability to rationally design future chemo-free strategies for lymphoma.
Why Precision Matching Matters
Trials like SYMPHONY-1 include complex eligibility — past therapies received, disease status, and specific regimen requirements. Without precise matching tools, patients can miss relevant trials simply because criteria are buried in unstructured text. CancerBot’s structured extraction logic can highlight eligibility in a way that traditional search can’t, connecting patients and physicians to trials they truly qualify for.
What About the Goodness Score?
As with all trials CancerBot rates the trial based on risk, benefit and patient burden. The risk is moderate (11 out of 20) as all of these drugs (tazemetostat, lenalidomide and rituximab) are well studied with well-known risks and clear protocols for monitoring them and addressing adverse events. The benefit is scored as just 3 out of 20, because, ESMO-MCBS’s rubric emphasizes benefits over the standard of care, which here is R² (lenalidomide plus rituximab). Until this study is completed the “benefit over alternative” cannot be fully assessed, so we must still score it low. The burden is fairly high, 15 out of 20, as R² already has a burden of 11. Adding another drug to be frequently taken increases that burden.
Bottom Line
SYMPHONY-1 is an important trial exploring whether adding a targeted EZH2 inhibitor to a standard immuno-therapeutic backbone can improve outcomes for patients with relapsed or refractory follicular lymphoma. Early signals are promising, and the ongoing Phase 3 portion will show whether this combination can set a new bar for therapy.






